UC Irvine is sparking the next revolution in batteries
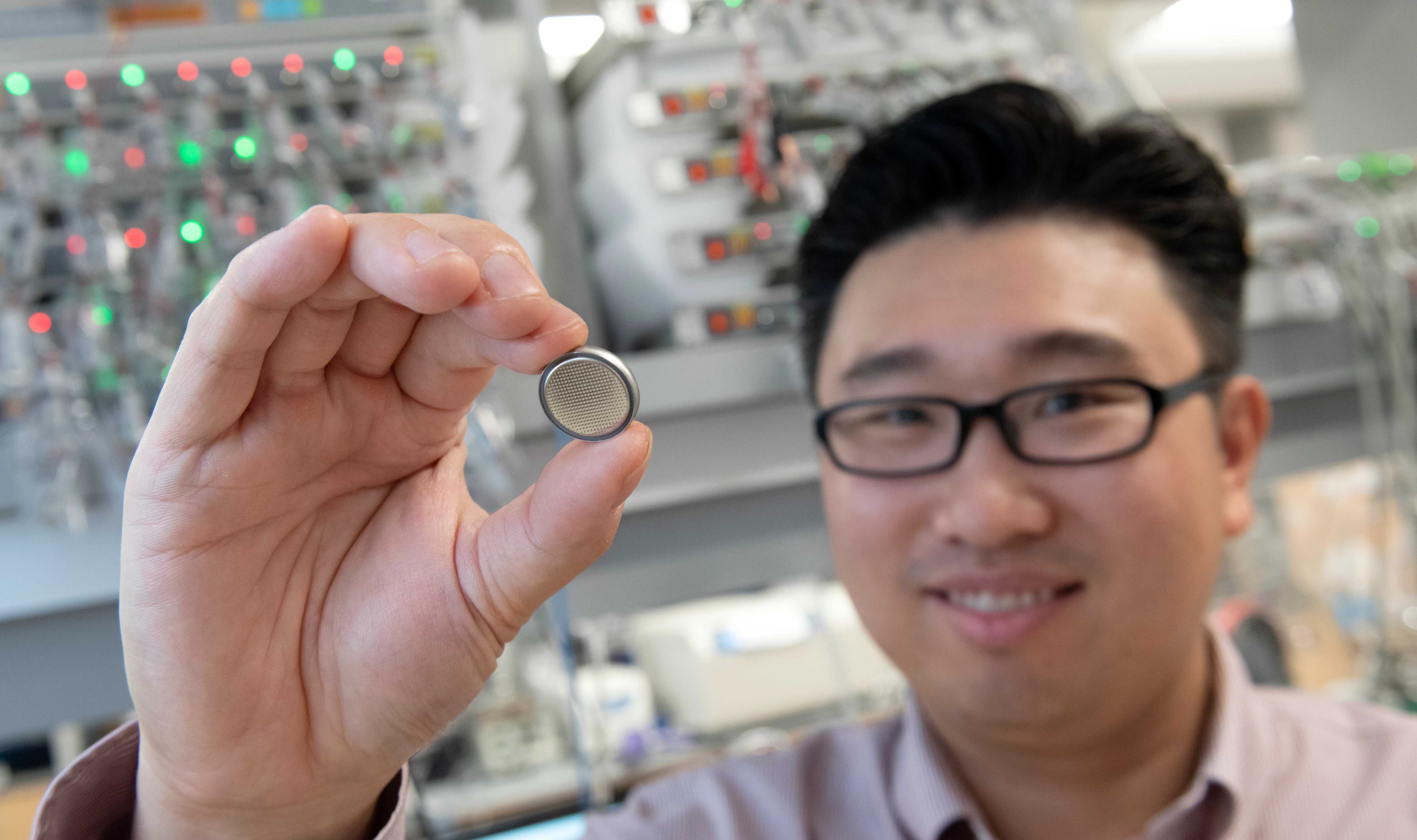
Professor Xin holds up a material created in his lab that promises to help push electric vehicles into a brighter, cleaner future.
Electric vehicles account for about 1.4% of all cars on the road in the United States. That figure continues to grow, but one thing keeping it from growing faster is what makes EVs possible in the first place: lithium-ion batteries.
Today’s lithium batteries require a critical mineral called cobalt, which helps the batteries store more energy. But the benefits conferred by cobalt come at a cost: about 70 percent of the world’s cobalt is mined in conflict zones like the Democratic Republic of the Congo where child labor is rampant.
“Cobalt is the new blood diamond,” said UC Irvine professor of physics Huolin Xin during a recent presentation about his lab’s battery research. “It’s highly valuable and dangerous to extract.”
Many drivers opt for an EV over vehicles with conventional internal combustion engines because the former is seen as having little-to-no negative impact on the environment. But, Xin explained, the ethical argument for buying modern EVs is not black and white.
Another issue with modern lithium-ion batteries is their safety. On average, about one out of every ten million lithium-ion batteries will catch fire, which can, in the worst cases, create situations that kill drivers and passengers.
Lithium batteries work because part of the battery is liquid – it needs to be in a liquid state so that the lithium ions can flow from one electrode to another inside the battery. But there’s a catch: at the right temperatures, that organic liquid can easily catch fire.
Lithium metal is special because it has a relatively high energy storage capacity. But until recently the only safe and cost-effective way of delivering lithium-ion batteries to market has been storing lithium in the form of ions in a non-metallic form.
For a lab like Xin’s, the task is clear: develop solid-state, cobalt-free lithium-metal batteries.
“I'm driven by the challenge of solving difficult problems – especially ones that others haven't been able to crack,” Xin said.
If you visit Xin's lab in the basement of Rowland Hall at the UC Irvine School of Physical Sciences, you’ll see experiments happening in what seems like every corner and on every shelf and every surface.
It’s clear that science is happening everywhere in Xin’s lab, but what's perhaps less clear is that in spaces that Xin’s research group occupies at the university, an energy revolution is underway. And that revolution, in this case, looks like a tiny silver coin.
On a visit to the lab, Xin held up the coin, which is a button-type battery developed by his lab that promises to solve the problems plaguing this generation's lithium batteries.
“It’s a magical material created in this room,” said Xin.
It’s magical because it’s a solid-state material that can successfully respond to the kinds of stresses and strains that conventional liquid-state lithium batteries are good at withstanding, and which other solid-state lithium batteries are currently unable to endure.
“I always wanted a material that’s soft, yet solid,” Xin said. “I want the interface to be squishy.”
The new material, because it’s a solid that retains material properties of something liquid, is able to “self-heal,” whereby, if a crack forms, the material will flow into and fill a crack. And it’s able to do this in real time as the battery is running.
Recent studies published by Xin and his team, including in Nature Communications, Angewandte Chemie and Advanced Materials describe the self-healing properties of the new solid electrolyte for lithium-metal batteries.
What’s more is that the material greatly reduces the need for the element nickel in lithium batteries; nickel mining can have a tremendous environmental impact, and, like cobalt, carries the potential for human rights abuses.
Now, Xin and his group are planning to work with entrepreneurs to take their new material to market, where he hopes it becomes a standard part of lithium-ion batteries.
“I want my work to contribute meaningfully to humanity through technological innovation,” said Xin.
