
Methane/Propane Double Hydrate
METHANE - PROPANE DOUBLE HYDRATE: STORAGE & TRANSPORTATION
 Use of gas hydrates as a possible storage medium for alternative fuels, such as methane (CH4) is looked at favorable over highly compressed, liquefied gas storage, or current transportation of gases. However, formation of methane clathrates (SI) requires extreme conditions of pressure and temperature ranging from 290-5,076 psi (2-35 MPa) and 273-288 K, respectively. We propose the production of the SII clathrate by stabilizing the larger cavities with propane (C3H8) at a relatively low pressure (25 psi) (0.172 MPa) and moderate temperature (273.1 K). This stable SII clathrate hydrate therefore contains the smaller cages available for occupancy by methane. In this way, methane can be stored in a clathrate hydrate (SII) at near ambient temperature and pressure conditions.
Use of gas hydrates as a possible storage medium for alternative fuels, such as methane (CH4) is looked at favorable over highly compressed, liquefied gas storage, or current transportation of gases. However, formation of methane clathrates (SI) requires extreme conditions of pressure and temperature ranging from 290-5,076 psi (2-35 MPa) and 273-288 K, respectively. We propose the production of the SII clathrate by stabilizing the larger cavities with propane (C3H8) at a relatively low pressure (25 psi) (0.172 MPa) and moderate temperature (273.1 K). This stable SII clathrate hydrate therefore contains the smaller cages available for occupancy by methane. In this way, methane can be stored in a clathrate hydrate (SII) at near ambient temperature and pressure conditions.
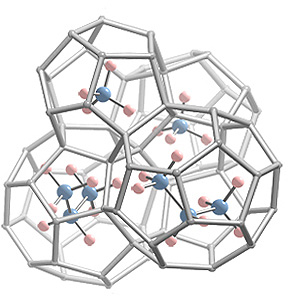
Propane hydrate is being studied to develop its use as a possible storage medium for methane gas. Decomposition temperature and pressure data are measured for the propane-methane hydrates. In order to use this data for feasible purposes, time efficiency is vital. The careful balance between good thermodynamics and effectiveness results in propane hydrates formed in short periods of time (72 hours). Approximately 68% of water is successfully converted to propane hydrate SII. The propane hydrates are then charged with varying pressures of methane gas. The resulting binary hydrates (propane-methane hydrates) are stable up to temperatures of 288 K and capable of filling up to 50% of the 512 cages, confirming propane hydrate’s capacity to store methane. Experimental propane-methane hydrate data are also used to generate enthalpy functions for Structure II hydrate for temperatures between 273 and 286 K.
Figure: Methane-Propane Double Hydrate (only 4 cages are shown)
Pressure-composition diagram of the mixed methane and propane hydrate system at 270.15 K, 275.15 and 278.15 K.
(from Ji-Ho Yoon et. al., AIChE, January 2004 Vol. 50, No. 1)
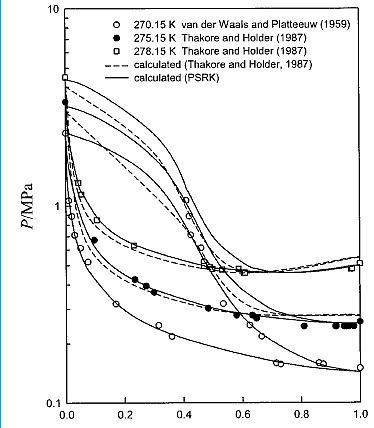
XC3H8
Figure 8. P-x Diagram for methane-propane hydratesystem at 270.15, 275.15, and 278.15 K.
Small molecules, such as H2S, may greatly enhance the stabily of methane-propane hydrate:
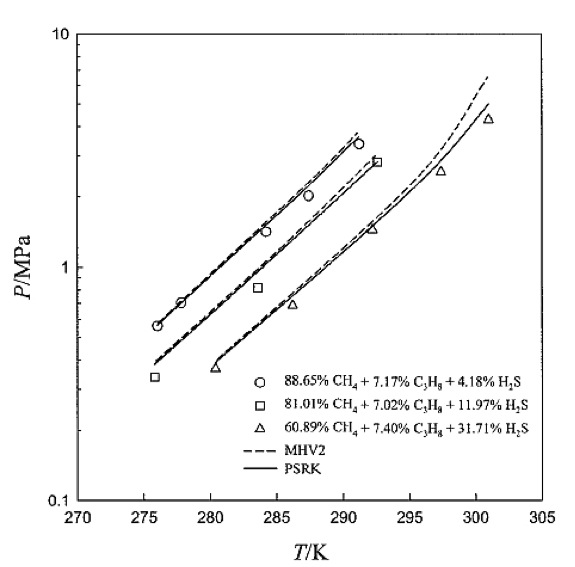
Figure 9. Dissociation pressures for methane-propane-hydrogen sulfide hydrates.
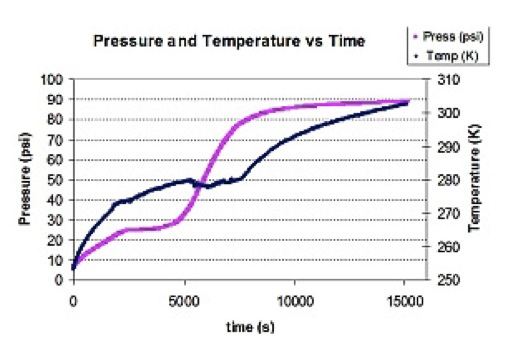
Temperature and pressure during decomposition of propane hydrate in a fixed volume.
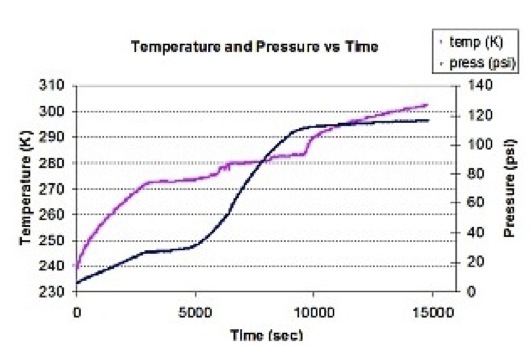
Temperature and pressure during decomposition of mixed (propane/methane) hydrate in a fixed volume. This binary clathrate was produced by first forming the propane hydrate and then pressurizing with 0.55 MPa (80 psi) of methane gas.


