
Resonance Raman
RESONANCE RAMAN SPECTROSCOPY OF HALOGEN CLATHRATE HYDRATES
/from Polymorphism in Br2 Clathrate Hydrates, Ilya U. Goldschleger, Galina Kerenskaya, Kenneth C. Janda, & V. Ara Apkarian, J. Phys. Chem. A 112, 787 (2008)/
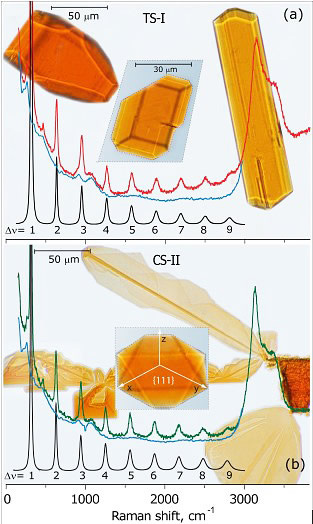
We have shown polymorphism in Br2 clathrate hydrates by identifying two distinct crystal structures through optical microscopy and resonant Raman spectroscopy on single crystals.
Single crystals of Br2 hydrate of the known TS-I structure (Fig.(a)) were grown from a saturated solution of Br2 and distilled water in a two-window cell at 4 °C. The crystals, which primarily belonged to the tetragonal bipyramidal class, had a melting point of 5.8 °C, consistent with the melting point of TS-I bromine hydrate. Cooling the sample below -7 °C initiated a rapid growth of new crystals (Fig.(b)) with very different morphologies. Single crystals grew either as thin plates, needles, or regular octahedra bound by {111} faces. This crystal habit is characteristic of CS-II clathrate hydrates, such as those of THF and Kr.
The two structures are clearly distinguished by the resonant Raman spectra of the enclathrated Br2, which show long overtone progressions and allow the extraction of accurate vibrational parameters: ωe= 321.2 cm-1 and ωexe = 0.82 cm-1 in TS-I; and ωe= 317.5 cm-1 and ωexe = 0.70 cm-1 in CS-II. These should be compared to the parameters of the free molecule, ωe=323.3 cm-1 and ωexe =1.06 cm-1 (note, that the anharmonicity is significantly lower for enclathrated bromine), and of the molecule in aqueous solution, where the fundamental appears at 306 cm-1 and no progression is observed. The RR spectra place the octahedral, platelet, and needle crystals in one class, distinct from the tetragonal structure. The spectral changes are clearly the result of differences in the cage structure and the cage-guest relative orientation, dictated by induction and dispersive interactions.
On the basis of structural analysis, the discovery of the CS-II crystals implies stability of a large class of bromine hydrate structures and, therefore, polymorphism.
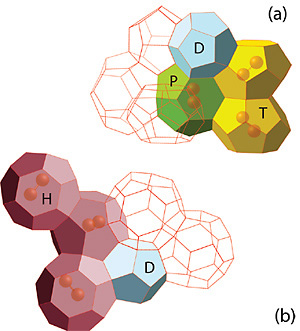
Figure: Parts of the polyhedral network in the TS-I (a) and CS-II (b) bromine hydrate structures. D (512), T(51262), P(51263), and H(51264) are standard notations for the convex polyhedra formed by hydrogen-bonded water molecules.


