Halogen Hydrates Spectroscopy
STRUCTURE OF BROMINE HYDRATE
/ References: 1. Konstantin A. Udachin, Gary D. Enright, Christopher I. Ratcliffe, and John A. Ripmeester,J. Am. Chem. Soc. 1997, 119, 11481-11486
2. Ilya U. Goldschleger, Galina Kerenskaya, Kenneth C. Janda, & V. Ara Apkarian, J. Phys. Chem. A 112, 787 (2008)/

One of the first discovered clathrates, Br2-clathrate hydrates have played a central role in our understanding of the stability of clathrates, and more generally, of solid solutions. The stacking of the Archimedean polyhydra of water, through hydrogen bonds, allows for a very large number of ways to tile 3-D space. Yet, not all possible structures are realized. Identifying the units that acquire special stability has been a challenge. Since its discovery in 1828 by Lowig, the structure and composition of Br2 hydrates has been controversial. The reported hydration numbers, which varied between 7 and 12, were previously assumed to arise from polymorphism, that is, more than one crystal structure. In 1997, the mystery of the bromine clathrate structure was believed to be resolved through single-crystal X-ray diffraction studies by Udachin et al. They concluded that “... different crystals of distinct compositions (Br2/8.62 H2O to Br2/10.68 H2O) and morphologies showed that there is just a single structure(tetragonal, P42/mnm, the structure originally proposed by Allen and Jeffrey) with considerable variation in the degree of occupancy of the large cages.”
We, however, have shown polymorphism in Br2 clathrate hydrates by identifying two distinct crystal structures through optical microscopy and resonance Raman spectroscopy on single crystals. Yet, bromine hydrate is the only example among gas hydrate that forms stable tetragonal structure. On a figure below is a general view of the bromine tetragonal 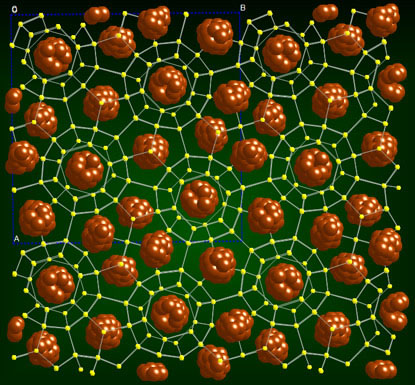 hydrate structure approximately along the z axis (hydrogen atoms are omitted). The cell has 172 water molecules that are hydrogen bonded to form the clathrate framework. The O...O distances vary from 2.69 to 2.90 Å, and the O...O...O angles between the hydrogen bonds vary from 100.6 to 122.3°. The water framework contains sixteen 14-hedral cavities 51262(T), four 15-hedral cavities 51263 (P), and ten dodecahedral cavities 512 (D) in the unit cell.
hydrate structure approximately along the z axis (hydrogen atoms are omitted). The cell has 172 water molecules that are hydrogen bonded to form the clathrate framework. The O...O distances vary from 2.69 to 2.90 Å, and the O...O...O angles between the hydrogen bonds vary from 100.6 to 122.3°. The water framework contains sixteen 14-hedral cavities 51262(T), four 15-hedral cavities 51263 (P), and ten dodecahedral cavities 512 (D) in the unit cell.
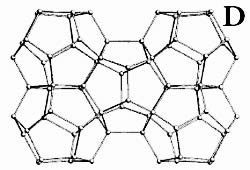
There are two distinct types of D cavities, which make up two five-D-cavity fragments (see below) typical of this structure. There are also two distinct types of T cavities per cell: 8 cavities that are connected with each other by hexagons and form columns along the c axis, and 8 which are paired along the 42 axis. The bromine molecules are located in the large T (51262) and P (51263)cavities while the small dodecahedral cavities are vacant or partially occupied by O2 or N2 molecules that are incorporated during crystallization in air.
When tetragonal bromine hydrate, in equilibrium with its mother liquor, is slowly cooled through -7ºC, a phase transition occurs. New crystals are formed on the surface of the tetragonal crystals, which grow at a much faster rate than the original. Based on morphology and resonance Raman spectra, the new crystalline phase can be identified to have cubic type II structure (CS-II).

Figure (right): Type II bromine hydrate crystals (light orange) growing from the surface of a tetragonal crystal (dark red). Dendrites of the type II clathrate hydrate structure are also shown.

