
Uv-Vis Absorption
UV-VIS ABSORPTION SPECTROSCOPY OF HALOGEN CLATHRATE HYDRATES
Dihalogen molecules are often employed as spectroscopic model systems due to their intense optical spectra and relatively easily assignable orbital symmetries. They serve as model systems for nonlinear optical studies, energy transfer studies, and cluster studies. Similarly, they are often used to observe how the local environment affects electronic structure, for example, in noble gas matrices, solvents,or microporous SiO2.
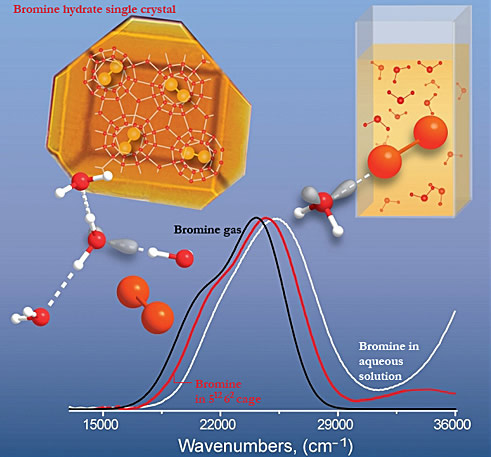
Here we use Uv-visible absorption spectroscopy to track changes in absorption spectra for halogens from the gas phase to clathrate hydrate environment and to liquid water/amorphous ice. For example, the absorption bands shift by about 360 cm-1 for bromine in large 51264 cages of type II clathrate, by about 900 cm-1 for bromine in 51262 cages of pure bromine hydrate, and by more than 1700 cm-1 for bromine in liquid water or amorphous ice. The shift and broadening in water and ice is due to the strong interaction of the water lone-pair orbitals with the halogen ó* orbital. In the clathrate hydrates, the oxygen lone-pair orbitals are all involved in the hydrogen-bonded water lattice and are thus unavailable to interact with the halogen guest molecule.
/Reference: Spectroscopic Signatures of Halogens in Clathrate Hydrate Cages. 1. Bromine, Galina Kerenskaya, Ilya U. Goldschleger, V. Ara Apkarian and Kenneth C. Janda, J. Phys. Chem. 110, 13792 (2006)/
|
Assuming εcb~1, Lambert-Beer Law: I0/I=exp(-εcb) Br2:H2O=1:7.5; ε=150 M |
 |
 The halogen clathrate hydrates optical density in a pure crystal is extremely high. To circumvent this issue, we make thin films of polycrystalline bromine clathrate hydrate and study them at temperatures where the halogen vapor pressure is negligible. Also, we studied low concentrations of trapped bromine molecules in type II hydrate cages formed with either tetrahydrofuran (THF,C4H8O) or methylene chloride (CH2Cl2) as the principle hydrate-former guest molecule. The THF or CH2Cl2 molecules occupy most of the larger cages, but in this study a small fraction of the large cages is occupied by bromine molecules(Section (B) on the above figure shows THF hydrate doped with bromine. Section (A) is bromine-THF-water melt) The halogen clathrate hydrates optical density in a pure crystal is extremely high. To circumvent this issue, we make thin films of polycrystalline bromine clathrate hydrate and study them at temperatures where the halogen vapor pressure is negligible. Also, we studied low concentrations of trapped bromine molecules in type II hydrate cages formed with either tetrahydrofuran (THF,C4H8O) or methylene chloride (CH2Cl2) as the principle hydrate-former guest molecule. The THF or CH2Cl2 molecules occupy most of the larger cages, but in this study a small fraction of the large cages is occupied by bromine molecules(Section (B) on the above figure shows THF hydrate doped with bromine. Section (A) is bromine-THF-water melt) |

Iodine In Type II Clathrate Hydrate
Iodine molecule is another halogen that is widely used as a probe of local environment. Even though iodine does not stabilize the clathrate hydrate lattice by itself, it has the appropriate diameter to fit into the 51264 cages of sII structure. We chose again to make double sII hydrate using the clathrate forming molecules THF, CH2Cl2 and CHCl3 with iodine as a second guest. The iodine concentration was always less than one percent of the hydrate forming molecule, and more typically 0.1 percent.

The figure shows the remarkable color change upon melting of the I2 doped THF clathrate hydrate. The rose-colored clathrate turns yellow as it decomposes. The process is reversible and the yellow iodine/THF/water solution turns rose again upon clathrate hydrate formation below 4.40 C.
UV-visible absorption spectrum of iodine in sII hydrate exhibits a relatively large, 1440 cm-1, blue shift. (In the gas phase or in some inert solvent iodine has a violet color, not rose). This is mainly ascribed to the differential solvation of the I2 electronic states. We conclude that iodine in sII hydrate resides in a 51264 cavity, in which the ground-state I2 potential is not significantly perturbed by the hydrate lattice. In contrast, in water and in ice, the valence absorption band of I2 is dramatically broadened and blue-shifted by 3000 cm-1. These observations are shown to be consistent with a strong interaction between a water molecule and iodine through the loan pair of electrons on water as in the case of bromine in the same media.
/Reference: Spectroscopic Signatures of Halogens in Clathrate Hydrate Cages. 2. Iodine, Galina Kerenskaya, Ilya U. Goldschleger, V. Ara Apkarian, Everyly Fleischer, and Kenneth C. Janda, J. Phys. Chem. 111, 10969 (2007)/


