
Gas Hydrates
GAS HYDRATES
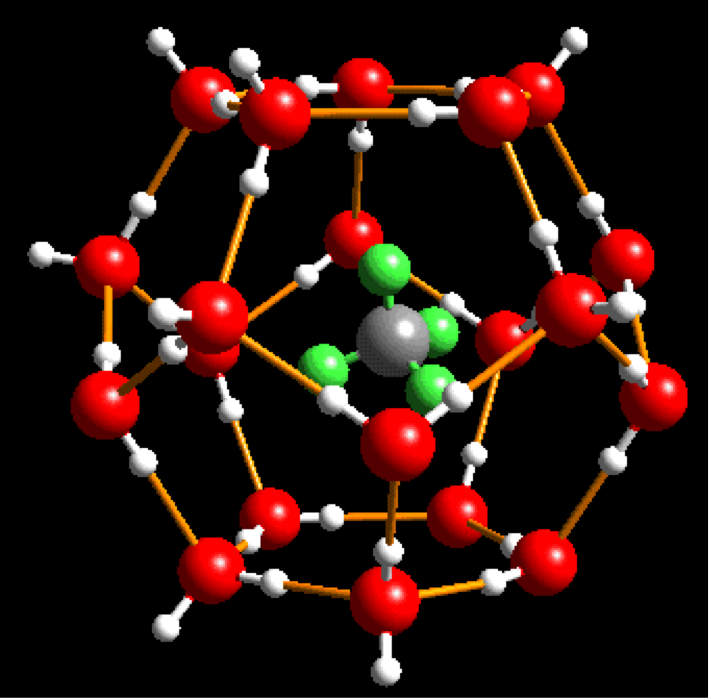
Clathrate hydrates (gas hydrates) are a ubiquitous class of crystalline inclusion compounds with nonstoichiometric composition, consisting of guest molecules trapped in a lattice of polyhedral water cages.
The resurgence of interest in this fascinating class of solids is, in part, motivated by the recognition of the vastness of natural deposits of methane hydrates and their potential global implications with respect to energy and the environment. Although the mechanism for forming these large deposits is not yet understood in detail, it is clear that methane in sediments combine with the water when the pressure and temperature are within the stability region. This occurs about 300 meters below the surface in temperate zones and close to the surface near the poles. An accidental release of all the deposited methane gas could cause a major climate change in a very short time.

Methane hydrate is also known to cause plugs in natural gas pipelines, an example of which is shown at left, (A huge pillar of solidified (natural) gas hydrate produced in a methane pipeline rupture). Knowledge of the nature of interactions that holds such structures together will provide a key to our ability to contain the methane hydrate or even use methane hydrates as a future energy source. Almost any molecule that is not too polar and fits into the clathrate water cage can form hydrates . Examples are oxygen, hydrocarbons, some ethers, halogens, CO2 and many more. Some gas clathrate hydrates can be stable almost up to a room temperature at 1 atm, for example Cl2 hydrate (discovered by Humphry Davy in 1811), others require a higher pressure and/or a lower temperature as methane hydrate.
 From Jeffrey and McMullan, The Clathrate Hydrates, 1967:
From Jeffrey and McMullan, The Clathrate Hydrates, 1967:
"The hydrophobic gases and liquids and the soluble acidogenic gases form hydrates when mixed with water at 0°C under pressures exceeding the invariant decomposition pressures. Crystallization takes place at the phase boundaries within the water, with the result that the gaseous or liquid dispersions become froths in which the bubbles of the guest species are encrusted with films of solid hydrate. If the reactive surfaces are renewed by agitation, the process is rapid but the product is micro crystalline and its growth slow, because there is no facile regenerative mechanism."
Unless precautions are taken to exclude atmospheric gases, they will also participate in the hydrate formation to a degree roughly proportional to their supernatant partial pressures. The inclusion of these gases can be recognized in the hydrates in which the other guest molecules are liquids by their evolution from the crystals on melting. Other gases which form these so-called "double" hydrates are H2S, H2Se, CO2, and the rare gases, with the probable exception of helium and neon. These are called "help gases" by von Stackelberg and Meinhold because their inclusion in the clathrate lattices enhances the stability of the structure, as shown by an increase in the decomposition temperature of the hydrate. From their observations on the preparation of many of these hydrates, von Stackelberg and Muller conclude that hydrophobic liquids having boiling points above 60 °C do not form hydrates, but in the presence of a help gas this upper limit can be raised so that it is virtually replaced by the size factor of the guest. Thus carbon tetrachloride with a boiling point of 77 °C does not form a hydrate by itself but does so in air of more than 2 atm pressure. This help-gas effect can be demonstrated very simply in the laboratory by passing H2S into a mixture of water and carbon tetrachloride at 5°C to give a white precipitate of the double hydrate.
 The preparation of single crystals of these hydrates is not readily achieved unless the guests are somewhat soluble above 0°C, as in the case of CO2, SO2, and N2O, where cubes or truncated cubes have been observed, and Br2, which forms tetragonal prisms (right) /tetragonal structure is one of the stable bromine hydrate polymorphs/. With less-soluble compounds, hydrate crystals with less-defined habits have been reported by growth from the initial micro-crystals by "aging" in contact with the liquid component near the decomposition temperature. The double hydrates of organic halides and H2S have been observed to form optically isotropic octahedra or hexagonal plates by sublimation.The preparation of single crystals of these hydrates is not readily achieved unless the guests are somewhat soluble above 0°C, as in the case of CO2, SO2, and N2O, where cubes or truncated cubes have been observed, and Br2, which forms tetragonal prisms (right) /tetragonal structure is one of the stable bromine hydrate polymorphs/. With less-soluble compounds, hydrate crystals with less-defined habits have been reported by growth from the initial micro-crystals by "aging" in contact with the liquid component near the decomposition temperature. The double hydrates of organic halides and H2S have been observed to form optically isotropic octahedra or hexagonal plates by sublimation.
The preparation of single crystals of these hydrates is not readily achieved unless the guests are somewhat soluble above 0°C, as in the case of CO2, SO2, and N2O, where cubes or truncated cubes have been observed, and Br2, which forms tetragonal prisms (right) /tetragonal structure is one of the stable bromine hydrate polymorphs/. With less-soluble compounds, hydrate crystals with less-defined habits have been reported by growth from the initial micro-crystals by "aging" in contact with the liquid component near the decomposition temperature. The double hydrates of organic halides and H2S have been observed to form optically isotropic octahedra or hexagonal plates by sublimation.The preparation of single crystals of these hydrates is not readily achieved unless the guests are somewhat soluble above 0°C, as in the case of CO2, SO2, and N2O, where cubes or truncated cubes have been observed, and Br2, which forms tetragonal prisms (right) /tetragonal structure is one of the stable bromine hydrate polymorphs/. With less-soluble compounds, hydrate crystals with less-defined habits have been reported by growth from the initial micro-crystals by "aging" in contact with the liquid component near the decomposition temperature. The double hydrates of organic halides and H2S have been observed to form optically isotropic octahedra or hexagonal plates by sublimation.
The water-soluble compounds generally form hydrate crystals simply on cooling aqueous solutions with approximately the compositions of the stoichiometric formulas. These solutions then become viscous and often supercool. Solutions of ethylene oxide, tetrahydrofuran, dimethylamine, trimethylamine, diethylamine, and isopropylamine have been observed to form very large transparent hydrate crystals on freezing without any special control of the experimental conditions. Under similar conditions, solutions of the butyl, amyl, and tertiary ethyl and propyl amines yield dendritic crystals. However, for ethanol hydrate and some of the amine hydrates, which melt incongruently, there are very narrow concentration ranges in which a pure crystalline hydrate can be obtained.
Like the hydrophobic guest components, these soluble liquids also form double hydrates with a third molecular species, such as H2S, which then functions as a help gas. Thus solutions of tetrahydrofuran, when cooled to 8°C, have been observed to absorb H2S at atmospheric pressure, forming a micro crystalline solid which grew into well-developed single crystals when left in contact with the mother liquor for several days at about 20°C. The double hydrates of acetone with argon, krypton, and xenon have been prepared from aqueous solutions at — 30°C under gas pressures of 300, 30, and 1 atm, respectively. Solutions of ethylamine, dimethylamine, n-butyl alcohol, and methyl ethyl ketone have also been reported to give somewhat less stable double hydrate crystals with these inert gases under pressure. Most hydrate crystals are observed to evolve gas on melting and it seems likely, therefore, that unless particular precautions are taken, air also functions to a greater or lesser degree as a help gas with the water-soluble guests in the same way as with the hydrophobic guest molecules."
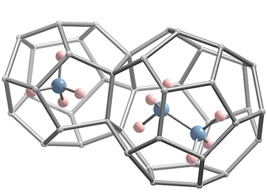
Example of methane-ethane double hydrate (only 2 cages are shown)


