


Next: About this document ...
Up: Lecture 2
Previous: Density of States Per
The total number of electrons
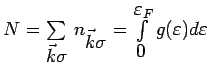 . We
could check our expression for
. We
could check our expression for
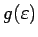 by plugging it in to see if
we get
by plugging it in to see if
we get 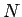 back. The total energy is
back. The total energy is
Now
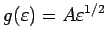 (It won't matter what
(It won't matter what 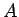 is.)
is.)
Thus
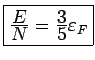
 (no
dependence on
(no
dependence on 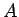 )
)
(Contrast w/ classical gas
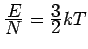 )
)
Pressure: Note
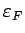 is proportional to
is proportional to
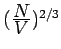 , i.e., it depends on
, i.e., it depends on 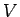 . If V is changed, the electron
gas should exert a pressure. At
. If V is changed, the electron
gas should exert a pressure. At 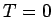 ,
,
Aside: Dimensions or units dictate
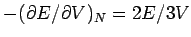 :
:
where
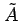 is a constant.
The inverse compressibility (or bulk modulus
is a constant.
The inverse compressibility (or bulk modulus 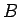 ) is defined as
) is defined as
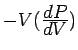 . (At
. (At 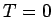 , the derivative is taken
with
, the derivative is taken
with
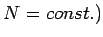 Since
Since
Using
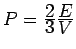 , we obtain
, we obtain
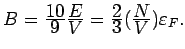
We would not expect 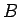 to represent the total bulk modulus (ions
contribute, too!), but the fact that this gives the right order of
magnitude means that the electronic contribution is substantial.
to represent the total bulk modulus (ions
contribute, too!), but the fact that this gives the right order of
magnitude means that the electronic contribution is substantial.



Next: About this document ...
Up: Lecture 2
Previous: Density of States Per
Clare Yu
2006-10-03
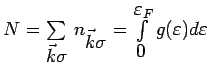 . We
could check our expression for
. We
could check our expression for
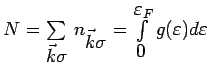 . We
could check our expression for
. We
could check our expression for
![]() by plugging it in to see if
we get
by plugging it in to see if
we get ![]() back. The total energy is
back. The total energy is
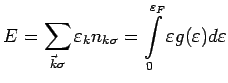
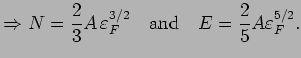
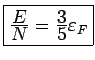
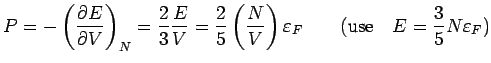
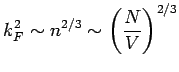
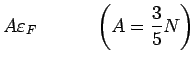
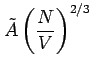
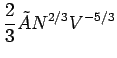
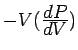 . (At
. (At 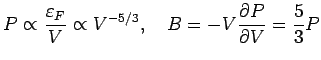
![]() to represent the total bulk modulus (ions
contribute, too!), but the fact that this gives the right order of
magnitude means that the electronic contribution is substantial.
to represent the total bulk modulus (ions
contribute, too!), but the fact that this gives the right order of
magnitude means that the electronic contribution is substantial.