


Next: About this document ...
Physics 224 Fall 2000
Discoveries and Inventions of Modern Physics due 11:00 am Tuesday Nov. 21
PROBLEM SET 8
Nov. 16 Colloquium:
``Reactions at Air-Water Interfaces in the Atmosphere: The New
Frontier in Atmospheric Chemistry''
Prof. B. Finlayson-Pitts, UC Irvine
3:30 pm, 101 Rowland Hall
Final Project
Reports on final projects will be given on Tuesday
December 5 from 10:30 am to 12:30 pm. Your oral presentation should
be 12 minutes long plus 3 minutes for questions (15 minutes total).
You may use the blackboard or transparencies in your presentation.
In addition you should hand in a written report that is 2 to 3 pages
long.
Problems
- 1.
- How many different meson combinations can you make with 1, 2,
3, 4, 5, or 6 different quark flavors? What's the general formula
for n flavors?
- 2.
- How many different baryon combinations can you make with 1, 2,
3, 4, 5, or 6 different quark flavors? What's the general formula for n
flavors?
- 3.
- The mass formula for decuplets of baryons assumes equal spacing
between the rows:
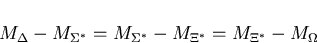 |
(1) |
Use this formula (as Gell-Mann did) to predict the mass of the
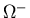 .
(use the average of the first two spacings to estimate
the third.) How close is your prediction to the observed value?
(See attached table of masses.)
.
(use the average of the first two spacings to estimate
the third.) How close is your prediction to the observed value?
(See attached table of masses.)
- 4.
- In the period before the discovery of the neutron many people
thought the nucleus consisted of protons and electrons, with the
atomic number equal to the excess number of protons. Beta decay
seemed to support this idea-after all, electrons come popping out;
doesn't that imply that there were electrons inside? Use the
position-momentum uncertainty relation,
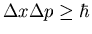 ,
to estimate the minimum momentum of an electron confined to a nucleus
(radius 10-13 cm). From the relativistic energy-momentum
relation,
E2-p2c2=m2c4, determine the corresponding energy,
and compare it with that of an electron emitted in, say, the beta
decay of tritium (a hydrogen atom with one proton and 2 neutrons)
(see attached figure). (This result convinced some people that the
beta-decay electron could not have been rattling around inside
the nucleus, but must be produced in the disintegraion itself.)
,
to estimate the minimum momentum of an electron confined to a nucleus
(radius 10-13 cm). From the relativistic energy-momentum
relation,
E2-p2c2=m2c4, determine the corresponding energy,
and compare it with that of an electron emitted in, say, the beta
decay of tritium (a hydrogen atom with one proton and 2 neutrons)
(see attached figure). (This result convinced some people that the
beta-decay electron could not have been rattling around inside
the nucleus, but must be produced in the disintegraion itself.)



Next: About this document ...
Clare Yu
2000-11-10
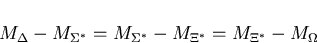
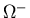 .
(use the average of the first two spacings to estimate
the third.) How close is your prediction to the observed value?
(See attached table of masses.)
.
(use the average of the first two spacings to estimate
the third.) How close is your prediction to the observed value?
(See attached table of masses.)
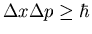 ,
to estimate the minimum momentum of an electron confined to a nucleus
(radius 10-13 cm). From the relativistic energy-momentum
relation,
E2-p2c2=m2c4, determine the corresponding energy,
and compare it with that of an electron emitted in, say, the beta
decay of tritium (a hydrogen atom with one proton and 2 neutrons)
(see attached figure). (This result convinced some people that the
beta-decay electron could not have been rattling around inside
the nucleus, but must be produced in the disintegraion itself.)
,
to estimate the minimum momentum of an electron confined to a nucleus
(radius 10-13 cm). From the relativistic energy-momentum
relation,
E2-p2c2=m2c4, determine the corresponding energy,
and compare it with that of an electron emitted in, say, the beta
decay of tritium (a hydrogen atom with one proton and 2 neutrons)
(see attached figure). (This result convinced some people that the
beta-decay electron could not have been rattling around inside
the nucleus, but must be produced in the disintegraion itself.)