
Janda Lab
Welcome to the web page for Ken Janda’s laboratory! Currently, my students and I are studying a class of solids called gas clathrate hydrates. In these species, 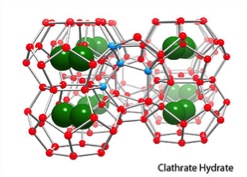 a water lattice is formed that creates cages, and these cages then enclose guest species that make the whole structure stable under certain conditions. Formally, these are called solid solutions since there is no formal chemical bonding between the guest molecules and the water lattice, and they are only stable as solid crystals. You may have heard about methane hydrates that are found in ocean and deep lake sediments and the arctic permafrost. They are one very important example of a gas clathrate hydrate. In the video above, we demonstrate the combustion of a propane hydrate clathrate. The sample looks like a cylinder of packed snow. However, when ignited, the ice lattice melts and the caged propane burns. The propane density in the sample is similar to that in a gas cylinder at 180 atmospheres of pressure.
a water lattice is formed that creates cages, and these cages then enclose guest species that make the whole structure stable under certain conditions. Formally, these are called solid solutions since there is no formal chemical bonding between the guest molecules and the water lattice, and they are only stable as solid crystals. You may have heard about methane hydrates that are found in ocean and deep lake sediments and the arctic permafrost. They are one very important example of a gas clathrate hydrate. In the video above, we demonstrate the combustion of a propane hydrate clathrate. The sample looks like a cylinder of packed snow. However, when ignited, the ice lattice melts and the caged propane burns. The propane density in the sample is similar to that in a gas cylinder at 180 atmospheres of pressure.
Gas clathrate hydrates are fascinating for both theoretical and practical reasons. The methane hydrates mentioned above could potentially be an important source of energy for our modern economy. Burning methane contributes to global warming, but much less than does burning oil or coal. By some estimates, there is enough methane stored in ocean sediments to power our economy for 500 years! Gas hydrates could also provide a safe method to ship methane and LPG around the world economically and safely. However, methane hydrate is also a potential source of disaster. Methane is a powerful greenhouse gas, more dangerous than carbon monoxide. If the natural methane hydrates are released as global warming progresses, the heating could accelerate out of control with drastic results.  The Deepwater Horizon disaster in the Gulf of Mexico in 2010 may well have been triggered by drilling through a methane hydrate deposit. Explore the “Gas Hydrates” link on this page to learn more about these issues.
The Deepwater Horizon disaster in the Gulf of Mexico in 2010 may well have been triggered by drilling through a methane hydrate deposit. Explore the “Gas Hydrates” link on this page to learn more about these issues.
Currently we have two active research projects. Several of my students are studying the kinetics of gas hydrate formation from ice particles and the potential guest gas. We have learned, for instance, that propane reacts much more quickly with small ice particles than with large ones.
For more on previous gas clathrate hydrate work that my students performed, please explore the links on this page.
The second current project is the one being performed in collaboration with Professor Martin’s group. We are using solid-state NMR (nuclear magnetic resonance) techniques to study the motions of water molecules in the hydrate lattice and guest molecules within the lattice. This work is still in the preliminary stages, and we need to get more results before publically discussing them.
A little background: I have always tried to choose research projects that are just beyond the ability of current chemical theory. The idea is to measure something that gives theoreticians a goal, and a test for the theories they are developing. For forty years I have been fascinated by the forces that hold molecules together and make them interact with each other. During this time, the ability of scientists to measure and compute the structure and dynamics of molecules has improved dramatically.
Finally, I would like to mention an experimental and theoretical tour-de-force that is a mostly unrecognized treasure. My students and collaborators performed spectroscopy on the iodine chloride molecule with 1 part per 500,000,000 resolution. They were able to measure the effects due to the fact that neither the iodine atom, nor the chlorine atom nuclei are spherical. This asymmetry allowed us to measure the asymmetry of the molecules’ electronic wave function as a function of the distance between the two nuclei. In essence, we measured the rehybridization of the electronic wave function as the molecule vibrates. I think it is fair to claim that this is the most detailed study of a heavy molecules’ wave function currently in the literature. To date, ab initio electronic structure theory has yet to be able to match these experimental results.
For a more complete and in depth introduction written by Dr. Kenneth C. Janda, click here.
 |
 |
|
Dynamical interrogation of the hydration cage of bromine in single crystal clathrate hydrates versus water Phys. Chem. Chem. Phys. 10, 2008 December, 2008. Cover |
Polymorphism in Br2 Clathrate Hydrates. J. Phys. Chem. A , 112, 5, p 787 February 7, 2008. Cover |


